Platform
1.1 Does the first domain need to be successful before a subsequent domain is started?
Funding constraints are preventing the start of other domains. If we secure additional funding or medications, other domains will start. It is not dependent on the success of the first domain.
1.2 Does a domain need to be complete before a subsequent domain is started?
No, participants can be enrolled into a new domain at any time.
1.3 Can a patient participate in two domains at the same time?
Yes, a patient can participant in more than one domain at the same time.
1.4 How can new staff members at sites be trained on trial procedures?
New staff can review SIV slides and study documentation. Regional Coordinating Centres can provide training, including refresher training, upon request.
2.1 Can patients be recruited if they do not tolerate ACEi/ARBs?
Yes, patients can be recruited as long as all standard of care therapies appropriate to their region have been offered to them.
2.2 Is current kidney transplant an exclusion criterion for the study?
While current kidney transplant is not an exclusion criterion for the platform, it is for the MRA domain. The exclusion criteria for the platform and individual domains are kept separate in the study.
2.3 Can patients receiving immunosuppressive therapy be recruited into the trial?
Yes, patients receiving immunosuppressive therapy can be recruited.
2.4 Can patients with high eGFR, for example eGFR > 85 ml/min/1.73m², be recruited into the trial?
Yes, they can be recruited. There is no upper cut off limit for eGFR.
2.5 Can the Screening visit and Randomisation visit be held on the same day?
The Screening visit and Randomisation visit can occur on same day provided that blood test and urinalysis test results are from different days. This requires the use of prior, standard of care pathology results (dated up to -31 days before randomisation) for the Screening visit. Blood and urinalysis tests for the Randomisation visit must occur on same day as randomisation. This is important as baseline eGFR and uACR is the average of 2 values from the screening and randomisation visits.
3.1 Are blood tests and urinalysis performed at local labs?
All pathology is performed at local labs and there are no central labs. Standard of care tests can be used if they fall within visit windows. For the randomisation visit, pathology will likely need to be performed for the trial as it is unlikely a test result is available from routine clinical care.
3.2 Can a patient remain in the trial if standard of care (SOC) treatment changes; for example, if the patient stops ACEi/ARB?
Yes, the patient can remain in the trial even if their SOC treatment changes.
3.3 Can a patient remain in the trial if standard of care (SOC) treatment guidelines change; for example, if a new treatment is considered SOC?
Yes, the patient can remain in the trial even if SOC changes. We will collect data on what treatments a patient is receiving at all visits.
3.4 Should the study intervention be stopped if patient commences on dialysis or has a kidney transplant?
Yes, the study intervention should be stopped if a patient starts on dialysis or has a kidney transplant. This is a study end point.
4.1 How will passive follow up be conducted?
We plan to follow patients every 5 years using medical records. Sites will be asked to check patient medical records to assess survival (dead or alive) and kidney (receiving dialysis or transplant) status. Data linkage may replace review of medical records in jurisdictions where it is available.
4.2 How are unknown dates entered into the eCRF?
If the exact day is not known, enter ’15’ for day. If the month is not known, enter ‘June’.
5.1 What is the primary outcome for CAPTIVATE and how was this selected?
The primary outcome for CAPTIVATE is chronic estimated glomerular filtration rate (eGFR) slope (mL/min/1.73m2/year) estimated from all available “on-treatment” eGFR values from week 4 to week 104.
Large trial data have demonstrated that eGFR slope is a valid surrogate endpoint in phase 3 randomised controlled trials in chronic kidney disease (CKD) (Levey et al. AJKD 2020). This allows for smaller sample sizes and shorter trial durations compared to traditional “hard” kidney outcomes such as kidney failure. Several therapeutic agents that may potentially improve kidney outcomes in CKD and will be tested in CAPTIVATE produce initial haemodynamic effects, resulting in an acute “dip” in eGFR which typically occurs within the first 4 weeks. To mitigate the risks of type 1 errors, one approach is to utilise chronic eGFR slope, i.e. exclude the values during the acute period. Another method is to use eGFR values from randomisation to the end of a washout period to evaluate total “off-treatment” slope. Extensive expert discussions, statistical analyses, and trial simulations have been conducted to reach this final decision to use chronic eGFR slope as the CAPTIVATE primary outcome and the reasons are as follows:
- The use of chronic eGFR slope ensures consistency between the primary outcome and interim analyses, as off-treatment washout values are not available for those undergoing interim analyses.
- Excluding values during the acute eGFR dip will reduce statistical complexities and risk for error as additional domains are added and combination therapies are studied.
- The required sample size for assessment chronic eGFR slope is smaller than that for total eGFR slope.
- Importantly, chronic eGFR slope has been demonstrated to be a stronger predictor of kidney outcomes.
- For additional sensitivity analyses, we have included total eGFR slope measured from week 0 (randomisation) to week 108 (end of washout) as a secondary outcome.
Reference: Levey AS, Gansevoort RT, Coresh J, Inker LA, Heerspink HL, … Levin A, Perkovic V, Zhang L, Willis K. Change in Albuminuria and GFR as End Points for Clinical Trials in Early Stages of CKD: A Scientific Workshop Sponsored by the National Kidney Foundation in Collaboration With the US Food and Drug Administration and European Medicines Agency. Am J Kidney Dis. 2020 Jan;75(1):84-104. doi: 10.1053/j.ajkd.2019.06.009.
7.1 How can a site access specific study visit eCRFs and logs in the database?
The table below is a guide on how to access study visit eCRFs and logs from the Participant Summary Page:
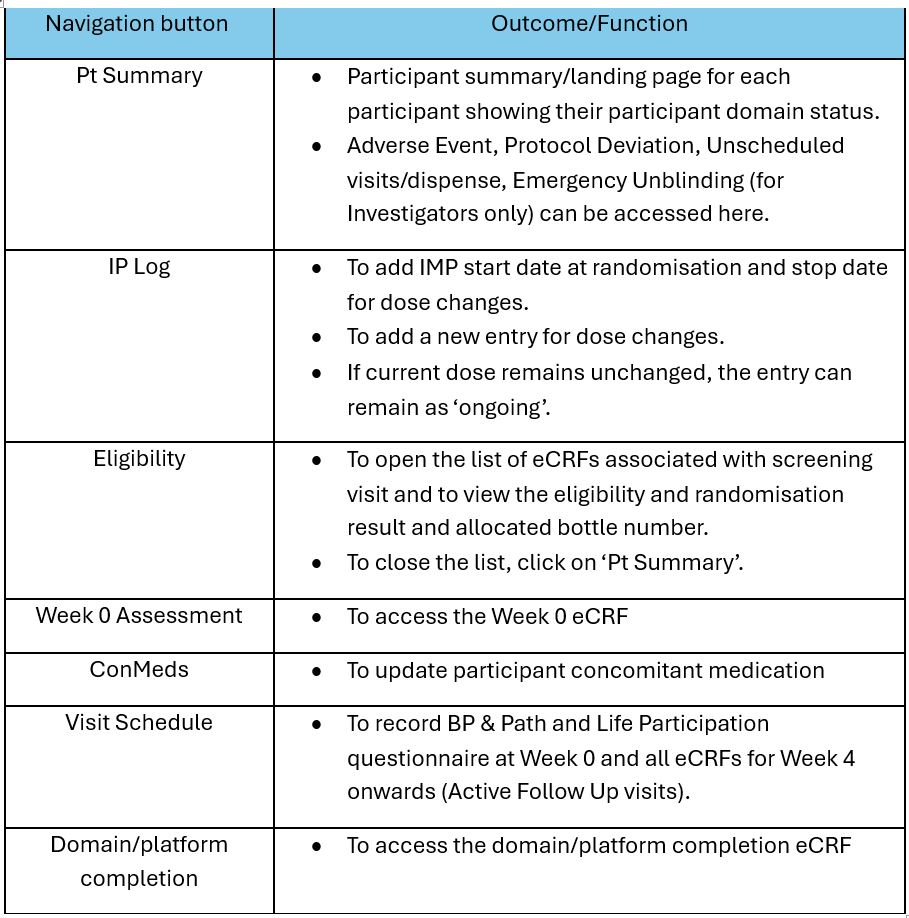
7.2 How can a site access the ‘Consent & Eligibility’ eCRF for participant that was previously pre-screened?
To access the ‘Consent & Eligibility’ eCRF for participant that was previously pre-screened, go to the participant’s pre-screening record and click on ‘Continue’. The ‘Start Eligibility’ button will appear if the participant is deemed eligible at pre-screening.
7.3 How can a site enter unknown dates in the eCRF?
- *Unknown Day*: If the exact *day* of the month is not known, enter *’15‘* for the day.
- **Unknown Month*: If the exact *month* is not known, enter *’June‘* for the month.
By following this guidance, the data will maintain consistency across the system while accounting for any uncertainties regarding dates.
7.4 How can study staff opt-in or opt-out to receive automated email notifications?
To opt-in or opt-out to receive automated email notifications, go to ‘My Details’ under your account and click on ‘Emails’. Select ‘√’ to receive email alerts or ‘X’ to opt-out.
MRA Domain
1.1 If a patient with hyperkalaemia is started on a potassium lowering agent to reduce serum potassium to below 5 mmol/L, is this patient excluded from recruitment into the MRA domain?
Provided potassium is <5 mmol/L at most recent blood test during screening period, patient is eligible for recruitment even if they are on a potassium lowering agent.
1.2 Is the use of a potassium binder an exclusion criteria for the MRA domain?
No, use of a potassium binder is not an exclusion criteria.
1.3 Bloods are usually taken on the day of randomisation, but test results come out later in the day (after the visit for randomization). In case K level is >5.5 mmol/L, does participant need to hold off commencing study medication and do another blood test in 1 week?
Yes. They should employ some potassium lowering strategies before the next blood test in a week. The finerenone guidance document contains more information about this.
1.4 The KDIGO definition for AKI is very strict, and patients are unlikely to have the frequency of pathology tests required to meet the KDIGO AKI definition. Can we use the clinical discretion to diagnose AKI?
The KDIGO definition must currently be used as per protocol. The CAPTIVATE team will consider whether the AKI definition should be loosened to allow more flexibility for site assessments, taking into account the impact on the volume of reporting. At the next protocol amendment, the CAPTIVATE team will propose the AKI definition is changed to ‘as per the KDIGO definition or at discretion of the investigator’.
2.1 Can the site select the number of bottles that are dispensed to supply the patient until the next visit?
The database will recommend the number of bottles, but the site can change this number.
2.2 Which staff members can sign-off eCRFs and emergency unblind?
Any personnel with the Investigator role in the database can sign-off eCRFs and emergency unblind. The site can choose which Principal Investigator and Sub-Investigators have Investigator role(s) in database. It is a good idea to have more than one staff member with the Investigator role for emergency unblinding purposes.
6.1 Are there any limits on the time period between pausing and allowing restart of study medication in the MRA domain?
No, there are no timelines or limits on when study medication can be re-started.
6.2 Do tablet counts have to be exact?
No. Approximate tablet counts are acceptable based on weight or visual inspection.
6.3 If finerenone/placebo is suspended due to K+ >5.5 mmol/L and then later restarted, should the drug be titrated up after re-starting?
Yes, finerenone/placebo should be titrated up after re-start if it is safe to do so. The aim is to achieve the maximum dose possible for as long as possible while maintaining patient safety. Finerenone/placebo can be up-titrated at any time if the patient meets the criteria for up-titration.
6.4 Will the database be allocating the correct drug bottles and will sites need to check the expiry date of drug selected?
The database should dispense the correct bottles and should not dispense bottles that are close to expiry. However, it is always a good practice for sites to double-check the bottles allocated and contact TGI immediately if there is a problem.
6.5 Can open bottles of finerenone/matched placebo be re-dispensed to the same patient after tablet counts?
Yes, opened bottles can be re-dispensed to same patient if the dose does not change and this is permitted by local regulations.
7.1 How can a site view the bottle number dispensed at an active follow-up visit?
The list of bottle numbers along with the dose dispensed at any active follow-up visit can be viewed by clicking on the Active follow-up eCRF specific to a study visit timepoint. The Investigational Product Dispensed section will appear at the top of the eCRF.
7.2 Can changes be made to Screening visit eCRFs after the patient is randomised?
Once the data entered in the Screening Visit eCRFs (Consent and Eligibility, MRA DSA Incl/Excl, Screening, Con Meds, BP & Pathology) is saved and the patient is randomised, the forms are locked and changes cannot be made by sites. If changes to these forms are required, please email captivate@georgeinstitute.org.au and cc RCC/study monitor.
Note: Only Screening and Week -4 BP and Pathology forms can be unlocked for editing post-randomisation.
7.3 Does the IP log need to be updated if the IMP dose has not changed?
No. If the IMP dose is unchanged, the IP log entry can remain as “Ongoing”.
7.4 How can IMP be dispensed at Wk 12 visit if the dose has not changed and only 2 bottles of IMP were dispensed at Wk 4?
If the dose of IMP remains the same at the Wk 12 visit, the “Unscheduled Dispense” function must be used 3 times to dispense 3 bottles of IMP.
